Abstract
Background: Pulmonary arterial hypertension (PAH) is a deadly disease without effective non-invasive diagnostic and prognostic testing. It remains unclear whether vasodilators reverse inflammatory activation, a part of PAH pathogenesis. Single-cell profiling of inflammatory cells in blood could clarify these PAH mechanisms.
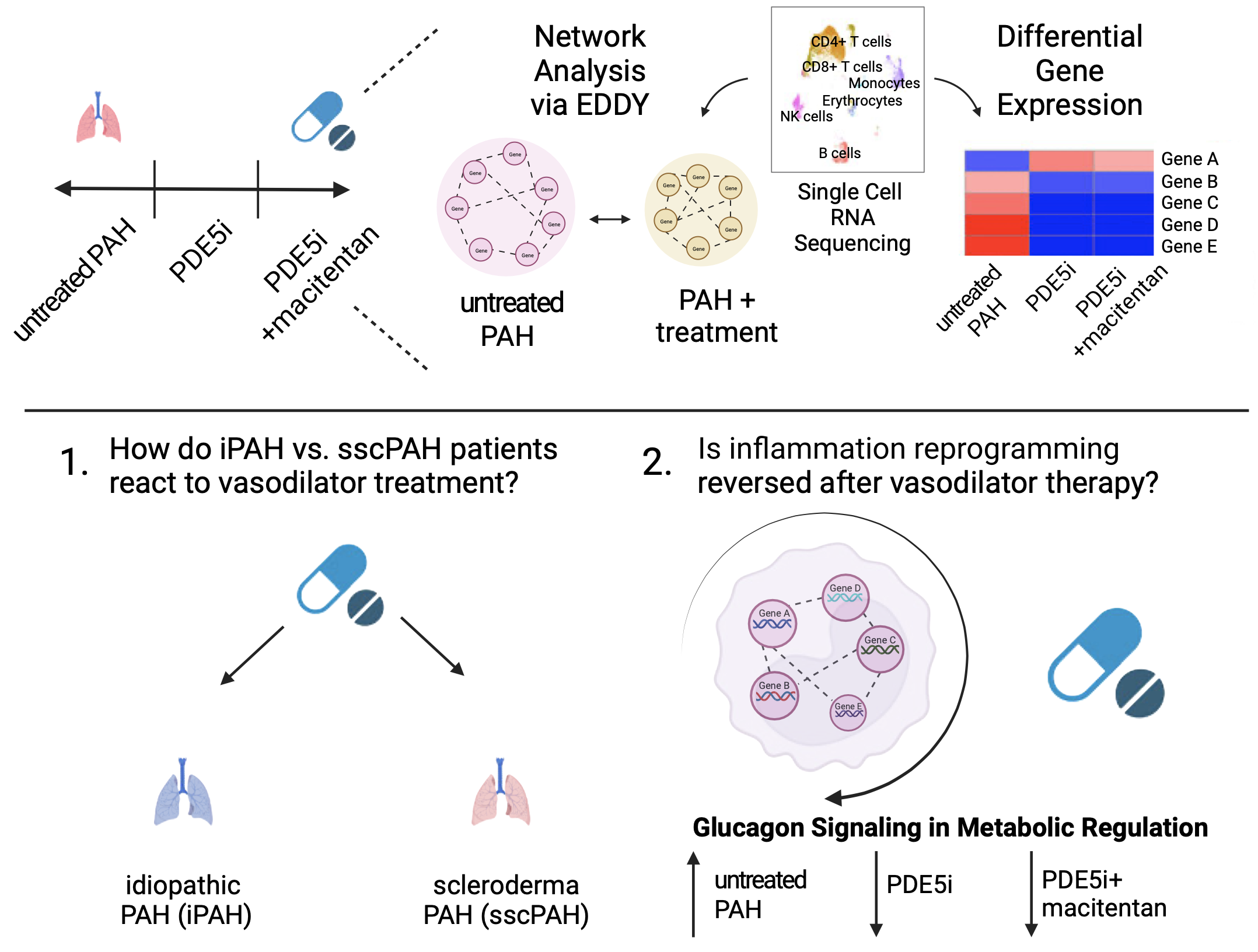
Methods: We evaluated a University of Pittsburgh Medical Center (UPMC) cohort consisting of idiopathic PAH and scleroderma associated PAH (iPAH and sscPAH) patients and non-PAH controls. We performed single cell RNA sequencing (scRNA-seq) of peripheral blood mononuclear cells (PBMCs) from controls (N=3) and PAH patients (iPAH and sscPAH), naïve to treatment (N=4), 3 months after phosphodiesterase 5 inhibitor (PDE5i) treatment (N=7), and 3 months after PDE5i+macitentan (N=6). We compared the transcriptomes of 5 PBMC subtypes from iPAH and sscPAH to observe their serial responses to treatments. Furthermore, we utilized network analysis to illuminate the altered connectivity of biological networks in this complex disease.
Results: We defined differential gene expression and perturbed network connectivity in PBMCs of PAH patients following PDE5i or PDE5i+macitentan. Importantly, we identified significant reversal of inflammatory transcripts and pathways in the combined PAH patient cohort after vasodilator therapy in every PBMC type assessed. The “Glucagon Signaling in Metabolic Regulation” pathway in monocytes was reversed after vasodilator therapy via two independent analysis modalities.
Conclusion: Via a systems biology approach, we define inflammatory reprogramming in blood of PAH patients and the anti-inflammatory activity of vasodilators. Such findings establish diagnostic and prognostic blood-based tools for tracking inflammatory progression of PAH and response to therapy.