Abstract
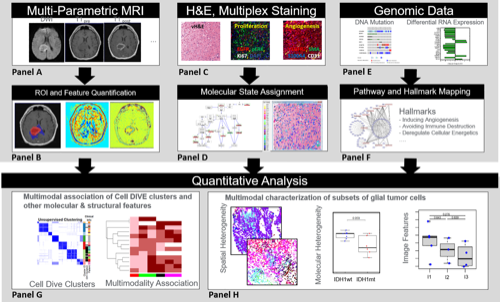
Glioma is recognized to be a highly heterogeneous CNS malignancy, whose diverse cellular composition and cellular interactions have not been well characterized. To gain new clinical- and biological-insights into the genetically-bifurcated IDH1 mutant (mt) vs wildtype (wt) forms of glioma, we integrated data from protein, genomic and MR imaging from 20 treatment-naïve glioma cases and 16 recurrent GBM cases. Multiplexed immunofluorescence (MxIF) was used to generate single cell data for 43 protein markers representing all cancer hallmarks, Genomic sequencing (exome and RNA (normal and tumor) and magnetic resonance imaging (MRI) quantitative features (protocols were T1-post, FLAIR and ADC) from whole tumor, peritumoral edema and enhancing core vs equivalent normal region were also collected from patients. Based on MxIF analysis, 85,767 cells (glioma cases) and 56,304 cells (GBM cases) were used to generate cell-level data for 24 biomarkers. K-means clustering was used to generate 7 distinct groups of cells with divergent biomarker profiles and deconvolution was used to assign RNA data into three classes. Spatial and molecular heterogeneity metrics were generated for the cell data. All features were compared between IDH mt and IDHwt patients and were finally combined to provide a holistic/integrated comparison. Protein expression by hallmark was generally lower in the IDHmt vs wt patients. Molecular and spatial heterogeneity scores for angiogenesis and cell invasion also differed between IDHmt and wt gliomas irrespective of prior treatment and tumor grade; these differences also persisted in the MR imaging features of peritumoral edema and contrast enhancement volumes. A coherent picture of enhanced angiogenesis in IDHwt tumors was derived from multiple platforms (genomic, proteomic and imaging) and scales from individual proteins to cell clusters and heterogeneity, as well as bulk tumor RNA and imaging features. Longer overall survival for IDH1mt glioma patients may reflect mutation-driven alterations in cellular, molecular, and spatial heterogeneity which manifest in discernable radiological manifestations.